The gastrointestinal (GI) tract is a major target in GVHD. Conditioning-induced damage and mucosal barrier disruption are important factors in GVHD, however therapies targeting these processes have not been identified. Glucagon-like-peptide 2 (GLP-2) is an enterocyte-specific growth factor produced by L cells that has regenerative potential in models of GI damage. Its impact on the mucosal immune system has not been elucidated. We sought to examine the therapeutic and immunologic effect of GLP-2 in murine GVHD.
We employed a major MHC-mismatched GVHD model (C57BL/6J → BALB/cJ). Mice were treated with 800nmol/kg/day of Elsiglutide (a GLP-2 analogue, provided by Helsinn) or vehicle beginning on D+1 for 30 days. Treatment with GLP-2 significantly improved survival and GVHD scores (Fig. 1A), while increasing small intestine mass and villi length (Fig 1B). GLP-2 also reduced T-cell infiltration into the jejunum (Fig. 1C).
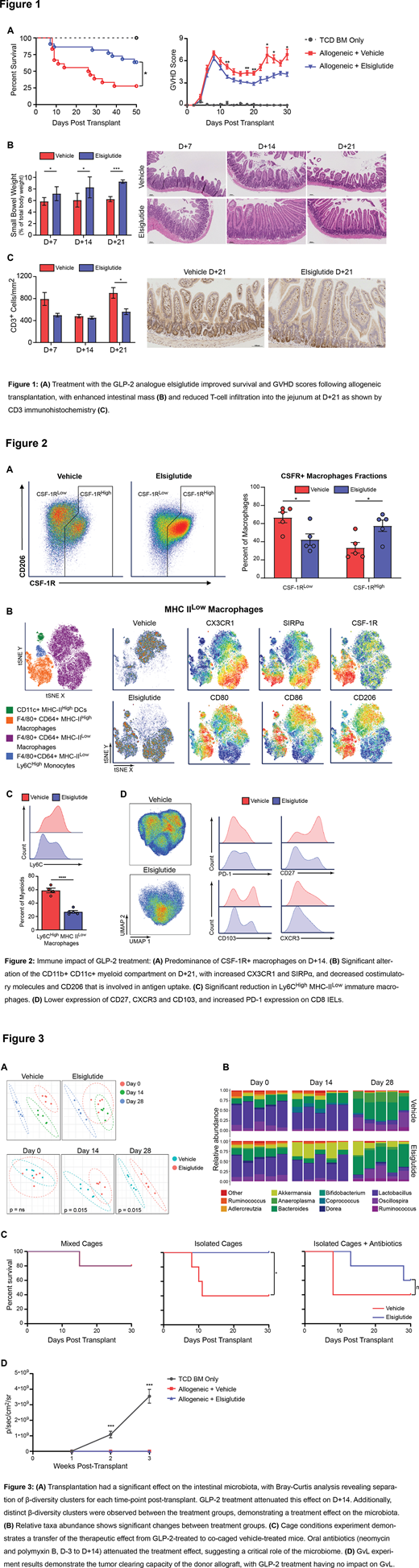
Analysis of intestinal immune cells by 28-color flow cytometry revealed dramatic differences between treatment groups in both myeloid- and T-cells. On D+14, GLP-2 led to an increased proportion of donor CSF-1R+ macrophages in the lamina propria (LP) (Fig. 2A) - cells that support the maintenance of the intestinal stem cell niche (Sehgal, Nat Commun, 2018). On D+21 the LP donor myeloid compartment was further altered, especially in MHC IIlow F4/80+ CD64+ macrophages (Fig. 2B, C). Here GLP-2 treatment expanded macrophages with lower expression of the co-stimulatory molecules CD80 and CD86 as well as the phagocytic marker CD206, whilst increasing the inhibitory molecule SIRPα, consistent with a tolerogenic phenotype. GLP-2 treatment also increased CX3CR1 expression on MHC IIlow macrophages with reduced Ly6C - a phenotype associated with physiologic macrophage maturation and linked to the resolution of colitis (Zigmond, Immunity, 2012). Vehicle-treated mice, conversely, had predominance of Ly6Chigh MHC IIlow LP macrophages reminiscent of an early infiltrating phenotype and near absence of mature macrophages, suggesting an impaired monocyte-macrophage transition that was restored by GLP-2. In addition, GLP-2 treatment led to significant changes in donor intraepithelial lymphocytes on D+21 (Fig. 2D), where CD8 T cells exhibited decreased CD27, CD103 and CXCR3 expression but higher PD-1, suggesting less activation.
To assess potential mechanisms for the differences in macrophage and T-cell phenotype, we examined the impact of GLP-2 on the intestinal microbiota. A syngeneic BALB/cJ model was used to explore the effects of GLP-2 independent of GVHD. Stool samples from D+0, D+14, and D+28 were subjected to 16S rRNA sequencing. Vehicle-treated mice had distinct β-diversity clusters at all time-points, showing a transplant effect on the microbiota (Fig. 3A). GLP-2-treated mice had near-complete cluster overlap between D+0 and D+14, suggesting attenuation of the impact of conditioning. GLP-2 treated mice were significantly enriched for Akkermansia muciniphila and Bacteroidales S24-7 family at D+14 and D+28 (Fig. 3B). These taxa have been associated with anti-inflammatory properties and A. muciniphila abundance is linked to epithelial mucin production, which is increased by GLP-2.
We then assessed the role of microbial communities in the protective effect of GLP-2 by conducting an allogeneic transplant with 3 caging conditions; 1) vehicle and GLP-2 treated mice caged together, 2) caged separately, or 3) caged separately plus oral antibiotics. We observed a clear cage effect where co-housing the treatment groups improved the survival of vehicle treated mice (Fig. 3C), suggesting transferal of the therapeutic effect via the microbiome. Antibiotic administration also dampened the beneficial effect of GLP-2. Finally, we conducted a GvL experiment by co-transplanting Luc-A20 and monitoring tumor progression via bioluminescence imaging. Both GLP-2 and vehicle-treated mice eliminated the tumor, whereas mice receiving T-cell depleted bone marrow showed tumor progression (Fig. 3D).
In summary, our results demonstrate high therapeutic potential of GLP-2 in GVHD. GLP-2 administration led to reduced mortality, modified the microbiome and altered the intestinal immune response to a more tolerogenic state. This novel mechanism sheds light on the role of the enteroendocrine system in maintaining gut homeostasis and sets the stage for therapeutic clinical trials.
Uhlemann:Allergan: Research Funding; GSK: Research Funding; Merck: Research Funding. Reshef:Gilead: Consultancy; Magenta: Consultancy; Novartis: Consultancy; Monsato: Consultancy; Atara: Consultancy; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal